Sunday, July 31, 2011
Sunday Spinelessness - Butterflies!
Just a couple of pretty pictures today, and what could be prettier than tropical butterfiles? There is a great butterfly house at the Otago Museum, and every time I visit I try and challenge myself to find another angle from which to photograph these beautiful creatures. Sometimes the butterflies themselves make that all too easy:
But my favourite one took a little more work, trying to approach a Heliconius while it surveyed the artificial jungle the musuem has created for the butterflies
Labels: butterfly, environment and ecology, lepidoptera, photos, sci-blogs, sunday spinelessness
Sunday, July 24, 2011
Sunday Spinelessness is putting its feet up today
Today is my birthday and a Sunday. So, I'm going to sleep in, ride my bike slowly around the peninsula (if the forecast snow doesn't evenutuate) then... well , then I'll probably have to do some work. But I'm not going to find time to write anything here. If, however, you need a fix on spinelessness to get through your Sunday then I think my post on the Scientific American guest blog should do nicely.
Labels: not even trying, sci-blogs, sunday spinelessness
Sunday, July 17, 2011
Sunday Spinelessness - What the... ?
Issac Asimov provided one of my favourite quotes about science
The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' (I found it!) but 'That's funny...
Scientific questions are almost never solved in eureka moments. Answers take time, planning, analysis and, finally, results from multiple studies made available for the scientific community to scrutinize. But there plenty of exciting moments in science, and there is nothing quite like the feeling of noticing something that doesn't quite fit with the way you think the world works. From there you can start spinning off theories that might explain the anomaly, and most excitingly, think how you might test those ideas against reality.
Something a little bit similar goes on in the life of a bug nerd. There are lots of creatures I'd love to see and never have. I've never seen a tardigrade, or a mantidfly [you really want to click that link, they're amazing] and reading Ted's blog means I'm forever looking out for one of our native tiger beetles. It's definitely a thrill seeing finally seeing some creature that you're read about and seen photos of, but it's at least as exciting to see something you didn't even know existed. For a bug nerd, "What the ..." is just as exciting a phrase as "A'huh", and that's exactly what I said when I turned some tree bark and found this spiky little guy:

Just as "That's funny ..." is a trigger to hypothesising and mentally developing a research proposal, "What the ..." is a precursor to reading searching, viewing and poking about in the hope of placing the strange creature into what we know about life. I have to admit I haven't got very far in this particular example. The thickest of those tufts mark the back end of the animal, and I'm pretty confident it's a beetle larva (you can make out two of the three legs on the near side of the body is this photo). Beyond that I'm a bit stuck, there are plenty of weevils with larvae that live under bark, but this doesn't look like any I've been able to find. Similarly, quite a few beetles in the family Dermestidae (called 'skin beetles' since some species are scavengers of animal corpses and some even specialise in eating hide) have spikey looking larvae like this one, but I can't find any helpful references for these beetles in New Zealand and don't know if there is a give away as to whether this animal fits in that family. If anyone knows what this is, or might be, I'd love to learn!
Labels: beetle, larvae, photos, sci-blogs, sunday spinelessness
Sunday, July 10, 2011
Sunday Spinelessness - The first animals (molecular biology)
It's time to wrap up this series of posts on the origin of animals. If you are just tuning in here, I've already decided that early fossils are utterly fascinating but really not much help to us and had a look at some modern organisms that might give us an idea about how the major steps towards multi-cellularity might have been achieved. Today, I'm going to zoom down another level, and see if molecular biology can tell us anything about the first animals.
Life is really just a very special kind of chemistry. The organisms that emerge from the bewildering number of inter-connected chemical processes that make life tick are so complex, so varied and so far removed from the things that chemists study that we had to create a whole other science, biology, to study them. For a long time biologists and chemists more or less got on with their own problems, but in the middle of the 20th century the progress each field had made created the opportunity to actually study the molecules that make life. The so called "molecular revolution" in biology has effected almost every sub-discipline within our science, and the tools and data that revolution created can be used to try and understand the origin of animals. Over the last decade or so scientists have sequenced the genomes of an increasingly number of species, and in between the big headlines created by creatures like chimps, mice and humans; we've been able to learn about sponges, cnidarians and placozoans. Those sequences can help us understand how these animals work at a molecular level, and they can also be used to recover the relationships between different groups of animals.
Scientists call the branching trees we create to represent the relationships between species 'phylogenies', and as someone who spends quite a lot of his time working on molecular phylogenies (have you seen The Atavism's logo...) I should really be saying that this is the best way to understand the origin of animals. But that's not really true. DNA isn't quite the wonder-molecule that CSI may have lead you to believe it is, and having DNA sequences available isn't necessarily enough for us to recreate a phylogeny. At the moment, the relationships at the base of the animal family tree are very hard to untangle. We know that a group of animals called the bilatarians (you, me, insects, fish, sea urchins, nematodes, spiders and, well, really every animal you are likely to name off the top of your head) form one group distinct from the other animals, and that these animals can be further subdivided based on the way their embryology plays out. But the relationships of the non-bilatarian animals - a grab-bag of creatures including the cindarians (jellyfish, corals and their relatives), comb jellies, placozoans and sponges - are really unclear:
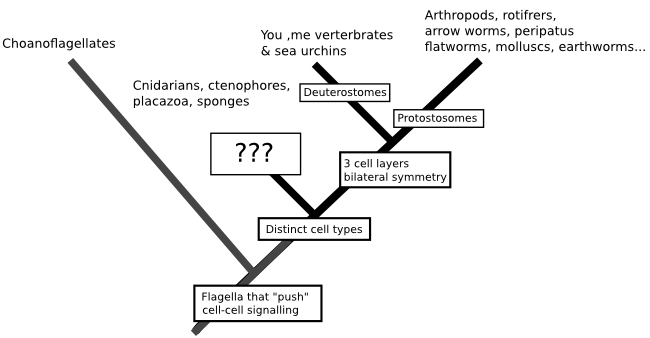
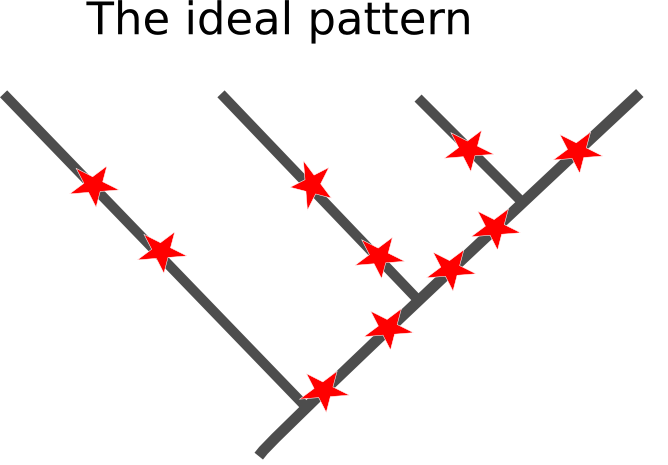
That uncertainty isn't a matter of us just not having enough data to throw at the problem. Molecular phylogeny works by grouping species together based on mutations that they share, having inherited them from a common ancestor. So, ideally all the branches in the true history of the species we are studying will be separated by enough time for those mutations to start racking up in the period of time that lineages shared an evolutionary history, something like this (with the mutations being the red stars occurring at random):
Sadly, the real pattern underling the base of the animal family tree doesn't appear to be like this. The initial branches fromed relatively quickly, leaving only short periods of shared ancestry for related groups to accrue mutations that would allow us to recreate their relationship. Worse yet, the branches that lead out from initial splits are really long, which means there has been millions of years for any useful mutations to have been over-written by more recent changes in DNA sequences.
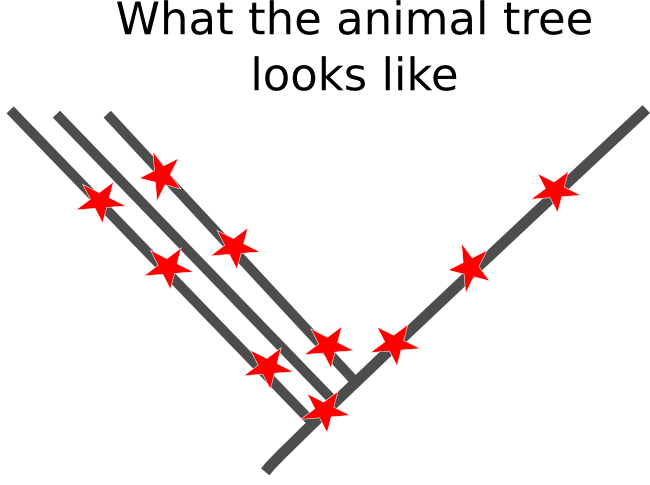
There is no easy way to get around this problem. It seems like we should be able to throw more and more genetic sequences at the tree, increasing the chance the we get some informative mutations, but these trees run the risk of falling into the terrifying Felsenstein zone, in which the confidence with which we estimate the wrong tree increases as we apply more sequences to the problem. Because of the difficulties inherent in reconstructing the very early history of animals, I think we should take results based solely on molecular phylogenies with more than a few grains of salt.
A case in point is the phylogeny Casey Dunn and colleagues presented in Nature a few years ago (doi: 10.1038/nature06614). If we were trying to fill in the question marks and the base of the animal tree without considering molecular data at all, we'd probably put sponges on the first branch to split in the animal family tree. Sponges are quite different from other animals, they don't have a nervous system, their cells are not bound into tissues and, although sponges do have specialised cell-types, their cells are uniquely flexible in that mature cells can change from one type to another. It's actually quite possible that several of the early branches in the animal tree lead to different types of sponges, like this:
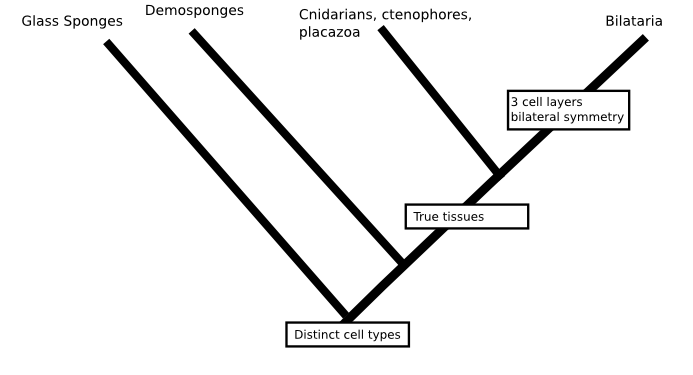 But, against all expectations, Dunn's phylogeny found comb jellies (creatures that look a little like jelly fish but form a phylum, Ctenophora, in their own right) to be the first group to split from the animal tree. If that were true, then we'd be less confident that the first animals were sponge-like since either (a) sponges would be radically modified animals which had lost their nervous system, given up on tissues and taken to a sedentary filter feeding life or (b) comb jellies would have evolved nervous systems and tissues independent of those of other animals.
But, against all expectations, Dunn's phylogeny found comb jellies (creatures that look a little like jelly fish but form a phylum, Ctenophora, in their own right) to be the first group to split from the animal tree. If that were true, then we'd be less confident that the first animals were sponge-like since either (a) sponges would be radically modified animals which had lost their nervous system, given up on tissues and taken to a sedentary filter feeding life or (b) comb jellies would have evolved nervous systems and tissues independent of those of other animals.
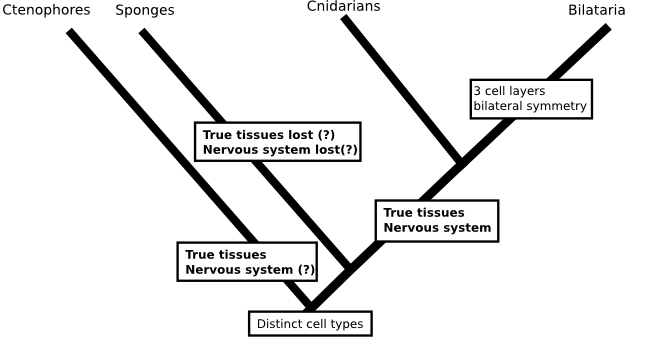
There is no reason that such a scenario would be impossible (in fact, fungi and plants have each evolved tissues independently of animals), but when the whole idea is constructed from a method that we know is prone to biases I don't think there is much point in worrying about it (I should say, this isn't a criticism of the paper I'm talking about, which took all the sensible measures to deal with the problems inherent in there analysis and presented their tree as something to work on rather than a final result). Similarly, a phylogeny that included data from everyone's favourite animals the placozoans (Schierwater et al., 2009. doi: 10.1371/journal.pbio.1000020) was used to resurrect a hypothesis for the origin of animals that includes a flat ancestor, not unlike a modern placazoan or the 'planula' larvae of some cindarians. This scenario would again require nervous systems to evolve twice:
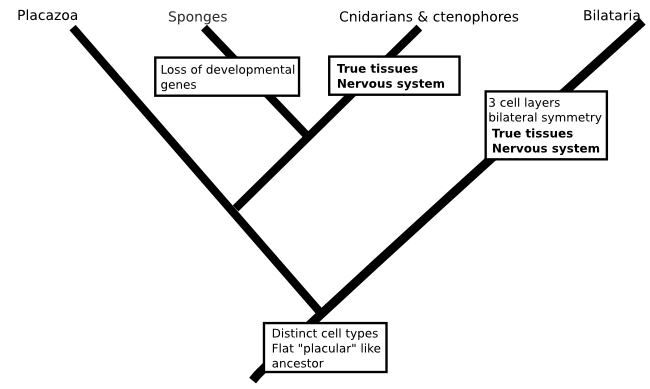
One of the reasons I'm skeptical of this idea comes from what we've learned about the genomes of simple animals. For my money, the discovery of Hox genes is the most remarkable result to come out of the the molecular revolution I talked about in the intro to this post. In turns out the genes that orchestrated your embryonic development have counterparts in Drosophila (or Sophophora if you'd rather) that do exactly the same job. If we move away from the bilataria, Hox genes are a bit harder to come by, but they still exist in the genomes of comb jellies, cnidarians and even palcozoans. But sponges have no Hox genes (Larroux et al, 2007. doi: 10.1016/j.cub.2007.03.008 and references therein). It's conceivable that the sponges descend from an ancestor that had Hox genes, then gave them up, but it seems much more likely that Hox genes evolved after sponges parted ways with the rest of the animal kingdom.
The contents of animal genomes tell us more than which phylogenetic patterns are likely - they can also let us know how some of the unique features of animals might have arisen. Almost every time the genome sequence of a simple animal is published you'll get a press release saying how surprising it is that genes associated with functions of more complex animals were found in this simple creature. And every time I read one of these press releases I sigh. Thesy are exactly analogous to someone that studies birds expressing surprise that therapod dinosaurs (the ancestors of birds) had forelimbs even though they couldn't fly. You can't make entirely new gene families out of nothing, new functions evolve when existing genes are retooled to attack different problems. The first animals faced two big problems when they moved from a single-celled lifestyle to a mulit-celled one: first, they needed to hold themselves together; and second, they needed ways for cells to communicate to their neighbours. Modern animals stick together with collagen (an entirely animalian invention) and a bunch of other sticky proteins including cadherins. The closest relatives of animals, the choanoflagellates which I talked about last week, have cadherins and other sticky proteins which they probably use to trap their prey (bacteria and other small cells).
Choanoflagellate genomes also give us a great insight into how the cells in the first animals started talking to each other. One of the most important pathways for cell-cell signalling in animals is called notch. Choanoflagellates don't have notch genes, but they do have the raw material from which one could be made. The proteins that notch genes make have several distinct 'domains' each of which perform particular function - and all these domains are present in choanoflagellate genomes! These different domains could have been brought together by genetic recombination, creating new genes by so called 'domain shuffling'. If we move just a little bit past the origin of animals and look at the genomes of sponges we can find the precursors of nervous system genes (see for example Liebeskinda et al., 2011. doi: 10.1073/pnas.1106363108).
So, that's three posts about 5 000 words and a whole bunch of figures on the origin of animals. What can we say we've learned? Certainly, studying modern animals seems to be the only way to approach the question of where animals came from. The models we looked at last week allow us to understand how the problems associated with a switch from single celled life, to a colonial lifestyle and finally the emergence of an individual identity for the colony. Modern genomes also tell us how those changes might have happened at the molecular level. Sometimes, as is the case of the cadherins and the evolution of the nervous system, existing genes could be re-purposed to a new tasks. Other times, new features were likely achieved with the help of new genes, cobbled together by genetic recombination. We can probably disregard theories for the origin of animals that require complex morphological changes based entirely on molecular phylogenies, since those estimates are prone to biases and the most interesting branches in them remain uncertain. If we look at morphological similarities between choanoflagellates and sponges (which goes right down to the collared and flagellated cells they use to eat) it seems likely that the first animals were filter feeders that pumped water into their cells with long flagella.
If you forced to make a bet on what the first animals were like, I reckon they'd be a bit like a sponge larva. A collection cells similar to a choanoflagellate colony, moving about in the water column with their flagella. The big question is how that individual could have emerged from the colony, and here I like Paul Rainey's ideas (described last week) about the way inter-cellular competition, which is bound to arise in a colony, could actually create the conditions that would foster cooperation. I'd be really interested to hear what any readers who made it all the way through this series think now.
Labels: animals, first animals, phylogenetics, sci-blogs, science, sunday spinelessness
Thursday, July 7, 2011
Our greatest journey
The Symphony of Science series of videos is probably the best justification for the continued existence of auto-tune. The latest of these videos, which put various scientists and science communicator's words to music, deals with the history of our species - the fact we all descend from African ancestors and that all populations outside Africa are the result of migrations that started around 150 000 ago:
It's a matter of continual disappointment and annoyance to me that most New Zealanders don't know that the person most responsible for finding our place in the biological world and locating the base of our family tree in Africa was a kiwi. Allan Wilson was a graduate of Otago University who went to Berkeley to use the new technologies that were developed for medical genetics to answer some of the oldest questions in biology. By sequencing DNA and looking at the cross-reactivity of immune system proteins, Wilson and his students established that humans only separated from other apes about 6 million years ago (as opposed to conformable distance of 25 million years paleontologists had favoured) and coined the term "Mitochondrial Eve" for one of the ancestors all modern humans can trace their origin to. This 'Eve' lived 150 000 years ago in Africa, so, just as Alice Roberts says in the video, we are all children of Africa.
These findings absolutely changed the way we think of ourselves as a species, and New Zealanders should know a kiwi was behind them! Thankfully the Royal Society has just announced one of Wilson's students, and a co-author on the most important papers, Rebbecca Cann is coming to New Zealand to talk about Wilson and his legacy. If you get a chance, I really encourage you to get along and hear her talk.
Labels: allan wilson, sci-blogs, science, science and society, science communication, video
Sunday, July 3, 2011
Sunday Spinelessness - The first animals (modern analogs)
The last time I tried to work out what the first animals might have looked like I decided fossils probably weren't much help. So, today I'm going stop looking back into the depths of time, and see if any modern creatures might provide clues as how animals got their start in life.
Remember from the last post, the major challenge for ideas about the origin of animals is explaining how a group of single celled organisms, each with their own evolutionary interests, can join together to create a mutli-cellular creature in which almost all of the cells can never reproduce in their own right. Our glance at earliest animal fossils record showed us that the resolution of this record just isn't fine enough for us to isolate the first cells to go in for this sort of arrangement, but there are a wealth of modern organisms that seem to have gone some way down this road, and they provide useful models for us to study.
Let's start by looking at the closest living relatives of animals the choanoflagellates:
 (photo is CC 3.0 from Choano-wiki (really!) user Mark J. Dayel)
(photo is CC 3.0 from Choano-wiki (really!) user Mark J. Dayel)
Choanoflagellates are a widespread a diverse group of single-celled creatures that live in the ocean as well as freshwater. At first glance it might seem a stretch to propose a relationship between these ten micrometre long cells and animals, but there is good reason to believe the relationship is real. Choanoflagellates, with their characteristic 'collar' around the tip of the cell body and the the flagellum extending from it are almost identical to a class of cells called choanocytes found in sponges. In fact, the two cells work in exactly the same way - the flagellum pushes water and nutrients into the cell body through collar were than are digested or, in the case of sponges, moved from one cell to another. By comparing molecular sequences, biologists have confirmed the choanoflagelletes are close relatives to animals, and also established they aren't simply a lineage discended of a sponge1 that gave up the multi-cellular lifestyle
The shared anatomy and feeding methods of sponge cells and choanoflagelletes gives us a clue as to how animals might have evolved. If a sponge is a bunch of cells that are held together by proteins that feeds using choanocytles, could the first animals have evolved from choanoflagellates that formed colonies? You don't have to imagine too hard here, because there are modern choanoflagellates that do just that:


In fact, colonial behaviour appears to have evolved multiple times within the choanoflagellates. This behaviour might crop up so often because even the solitary species have a wealth or sticky proteins that they use to trap bacteria and other food items in their collars. However it arises, colonial behaviour is obviously worthwhile for some choanoflagellates because they been doing it for millions of years, either forming spheres like the Sphaeroeca shown above, clusters like Protero below or as small groups sitting on a stalk like Proteospongia 2
Colonial choanoflagellates might well have been the first step on the road to true mulit-cellularity, but an agglomeration of cells each doing well out of their association with each other is still a long way from the specialisation we see in modern animals. Thankfully, there are organisms out there that give us a glimpse as to what the next step might have looked like. And some of them are stunningly beautiful:
 (photo is CC 2.0 from Wellcome Images)
(photo is CC 2.0 from Wellcome Images)
The sphere you see above us an alga called Volvox that makes blurs the line between a colony of single celled organisms and a multi-cellular life form. Of course, algae are only very distantly related to animals, but we are looking for models of how simple multi-cellular life might work, and Volvox is interesting because it's a very simple organism that has a clear distinction between reproductive cells and the rest of the organism. The closest realtives of these beautiful creatures are single celled algae called Chlamydomonas. Most of the time Chlamydomonas are free swimming cells, propelled about in search of sunlight for photosynthesis by two flagella:

When it comes time for them to divide they draw their flagella in and begin a series of cell-divisions, keeping between two and eight daughter cells within the 'old' cell wall before they burst out and get back to the swimming lifestyle

Volvox has ditched this two-stage life cycle, instead, the individual (or colony if you'd rather) simultaneously contains reproductive and 'swimming' cells. That dotted sphere is made up of thousands of cells very similar to swimming Chlamydomonas each connected in an extra-cellular matrix of proteins and carbohydrates. Importantly, those outer cells don't divide. Reproduction is down to a set of immobile cells within the sphere, called gonidia. Each gonidia can go through a set of programmed cell divisions that create all the cells that make up a new Volvox individual. Volvox is probably the simplest example of an organism that displays a division of labour between 'body cells' (in this case the swimming cells that move the individual around) and reproductive ones. As I said, algae are not closely related to animals, but the larvae of some sponges seem in some ways analogous to an individual Volvox. Like all sedentary animals, sponges have larvae that can move, and in sponge larvae that movement comes courtesy of a set of ciliated cells that form the lower portion of the larva:
A glass model of a sponge larvae, photo provided by Welsh Museum
So, between Volvox and sponge larvae we have an idea of what a very simple free swimming animal with specialised cell types might look like. But how might that division of labour between different cell types have evolved? Now we really are heading into some murky waters. Animal multi-cellularity happened once, at least 600 millions ago. Obviously any answer we offer as to why this happened is going to be at best a tentative explanation, but I've always like an idea developed by New Zealand evolutionary biologist Paul Rainey (and not just because he has been the head of a Centre for Research Excellence of which I'm a member!)
Rainey is an experimental evolutionary biologist, taking advantage of the speed at which miroogranisms reproduce to answer questions those of us that wander about in the field couldn't even begin to ask. One of his experiments involved growing bacteria in a stable environment, which reliably procudes mutants that are rather charmingly called "wrinkly spreaders". The wrinkly spreaders form mat-like colonies on the top of the tubes that they live in:

To be part of that mat each cell has to pay a small cost in the form proteins that stick the cells together, but that cost is more than repaid by the fact only cells in that mat can access oxygen from the barrier between the fluid in the cell and air above it. For this reason wrinkly spreaders soon take over the population in the tubes. Natural selection acts on individuals, not colonies, and very often selection acting on cells within the mat will lead to its destruction. Cells within the mat can take advantage of their neighbours by not producing the adhesive proteins that hold the mat together while still enjoying the benefit of being within it. In time, the small advantage these mutant cells gain by not paying the price in adhesive proteins will be enough to see them out compete their neighbours. But, of course, once such 'cheating' cells predominate the mat won't be able to sustain itself and it will fall apart.
Here's were is gets really interesting. Each mat seems like an evolutionary dead end, because the mats themselves can't reproduce (a prerequisite for evolution by natural selection) - when the mat falls apart the cells fall into the oxygen-free zone and die. But 'cheating' cells can reproduce and they can leave the mat and, most remarkably, because there are so many cells in a population that, in time, it's likely one of them will mutate back to the co-operative wrinkly spreader type. Now stand back and think about the big picture here. You have a larger stationary structure, the mat, that can give rise to small, mobile cells (the cheaters) that can each go on to establish a new large structure. That sounds very similar to a larval-adult life cycle, or even the distinction between body cells and reproductive cells that we are trying to explain. Since the 'cheater' cells probably arise by mutations that break existing genes, the switch between cheater and wrinkly spreader could, in time, be controlled by gene expression rather than by waiting for mutations.
Of course, I'm not trying to argue that animals evolved from wrinkly spreaders specifically, or even this sort of pattern generally. The really neat idea in Rainey's description of the dynamics of wrinkly spreaders is the way the cohesive nature of multi-cellular organisms might have evolved from competition rather than co-operation. Hundreds of co-operative systems have been identified within colonies and populations of single celled organisms and all of them are prone to sabotage by cheaters, so it's definitely something to think about, but, like all the ideas in this field, it is speculative and may turn out to be wrong.
So, modern organisms can give us a few clues as to animals might have got their start. Colonial choanoflagellates are an example of how simple colonies that feed in the same way as modern sponges could form. Volvox is an example of a very simple organism that has a distinction between reproductive and body cells and Rainey's wrinkly spreading bacteria show us one possible route to how that distinction would arise in the first place. In this peice of really presumed that the first animals fed in much they way modern sponges do, but not everyone thinks is the case. Next week I'll turn to genes, genomes and the family trees we can estimate from them to explain some of the slightly more outre ideas about the origin of animals.
1 I really wanted to call this post "To be descended of a sponge", but I called the first "The first animals" so I guess I'm stuck with it for the series
2 The world's number one protist fan, Psi Wavefunction, would like it to be known that the Proteospongia species you might read about that is meant to have specialised cell types similar to a sponge's ameboid cells probably doesn't exist (being recorded in error a in the 1880s and not seen since)
Lots of references today: Choanoflagellate biologists have their own wiki, and it's pretty cool The free online version of Molecular Biology of the Cell has a section on the evolution of multi-cellularity including Volvox as does Scitable, Nature's education website.Labels: animals, evolution, first animals, sci-blogs, science, sunday spinelessness





